Introduction: iron metabolism is deeply related to the pathogenesis and prognosis of acute myeloid leukemia (AML) and remains largely unexplored in the era of MRD-driven therapy. Several studies have assessed prognostic implications of altered iron-related laboratory values at diagnosis and there is growing interest towards the inherent pathobiology of iron in AML since new therapeutic strategies (i.e. targeting ferroptosis) are being investigated. Nonetheless, no clear association has been established between a specific risk subtype of AML within the new ELN2022 classification and a metabolic iron signature at diagnosis. Furthermore, data correlating an altered iron profile at diagnosis with post-induction response, rate of infections and need for PRBC transfusions are lacking.
Methods: We performed a retrospective monocentric analysis in intensively (IC) and non-intensively (NIC) treated AML patients. Effects of altered iron parameters at diagnosis (at least one between ferritin and transferrin saturation [TSAT] according to local-lab reference range) were investigated sub-dividing patients into an altered (AC) vs a non-altered cohort (NAC). 110 AML patients diagnosed between June 2019 and April 2023 were screened for this study. 62 patients were eligible: 46 IC (AC:22 vs NAC:24) and 16 NIC (AC:12 vs NAC:4) patients were analysed. 48 patients were excluded because of previous PRBC transfusions (if > 5 PRBC), active infection at diagnosis or severe comorbidity. We compared the two cohorts in terms of ELN22 risk classification, frequency of MDS-related mutations using an NGS-based panel, TP53 mutation rate, and rate of complex karyotype (CK). Furthermore, we compared AC vs NAC (both in IC and NIC) in terms of overall survival (OS), morphologic complete response (CR) rate, rate of post-induction measurable residual disease negativity (both PCR-based and MFC based), rate of G4 infections and PRBC transfusions during induction. In addition, ferritin and TSAT cut-off values to predict CR and MRD response were calculated using ROC analysis. Selection bias was assessed considering age, risk category, PRBC count before therapy, therapy regimen, comorbidity using Charlson comorbidity index (CCI) and CRP values.
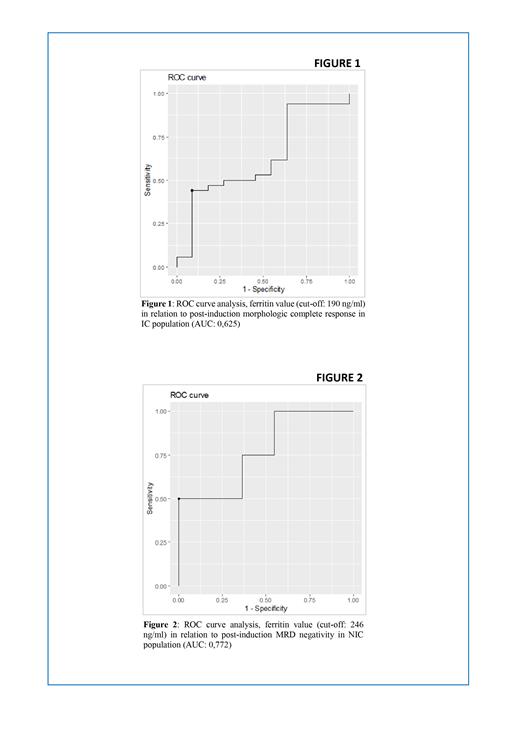
Results: There was not a significant difference in terms of high risk subtype frequency between AC vs NAC group in both the IC and NIC population (IC p: 0,39/NIC p: 0,29). In both macro-groups, the AC group did not show a significantly higher frequency of CK (IC p: 0,66/NIC p: 0,84), TP53 mutations (IC p: 0,91/NIC p: 1) and MDS-related mutations (IC p: 0,76/NIC p: 0,14). OS was not significantly different in the AC group in both IC and NIC (IC p: 0,16/ NIC p: 0,62). No significant differences were found between the two cohorts in terms of post-induction CR rate (IC p: 0,6/NIC p: 0,25), MRD-negativity rate (IC p: 1/NIC p: 1) and rate of G4 infective complications (IC p: 0,5/NIC p: 0,59). In the IC patients with high risk disease, an altered iron profile at diagnosis correlated with a higher need (>10) of PRBC during induction compared to high risk patients with normal iron profile (p-value 0,014). Iron-related lab values at diagnosis were informative in predicting post-induction CR and MRD status in both the IC and NIC population. In the IC population (figure 1) a ferritin cut-off value of 190 ng/ml (AUC: 0.625) predicted CR rate while a TSAT cut-off value of 48% (AUC: 0,587) was informative about MRD negativity rate. Similarly, in the NIC population a ferritin cut-off value of 246 ng/ml (AUC: 0,772) was informative about MRD negativity rate (figure 2).
Conclusions: Our retrospective study did not find an undisputable correlation between a specific ELN2022 AML risk subtype or gene-mutation using NGS and altered iron lab-values at diagnosis, whereas an association in ELN2022 high risk IC patients regarding PRBC transfused during induction in AC vs NAC was found. Although the AC did not exhibit a shorter OS compared to the NAC in both IC and NIC population, specific iron-related cut-off values were informative about disease response to both intensive and non-intensive treatments, including MRD negativity rate. Larger clinical trials to dissect iron-metabolism in AML pathogenesis, diagnosis and treatment are highly warranted.
Keywords: Acute Myeloid Leukemia, iron metabolism, ferritin, transferrin saturation, ELN22, MDS-related gene mutations, NGS, induction therapy, MRD, OS, transfusion rate
Disclosures
Papayannidis:Pfizer, Astellas, Janssen, GSK, Blueprint, Jazz Pharmaceuticals, Abbvie, Novartis, Delbert Laboratoires: Membership on an entity's Board of Directors or advisory committees; Abbvie, Astellas, Servier, Menarini/Stemline, BMS, Pfizer, Amgen, Janssen, Incyte, Novartis: Honoraria. Cavo:AbbVie: Consultancy, Honoraria; GlaxoSmithKline: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Sanofi: Consultancy, Honoraria; Celgene/Bristol Myers Squibb: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Adaptive: Honoraria; Roche: Honoraria. Curti:Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal